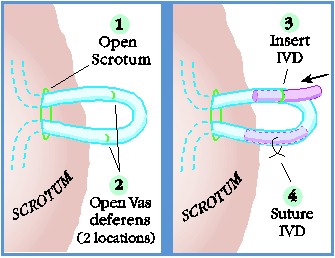
 |
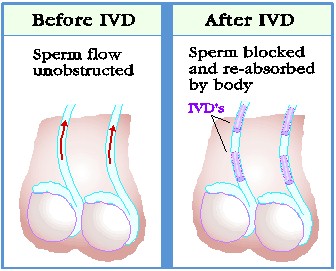 |
After the procedure, your doctor may have you lie down at home for a couple of hours with an ice pack on your scrotum. This will help to reduce any bruising, swelling and discomfort. These symptoms should subside within a few days.
It usually takes several months after a vasectomy for all remaining sperm to be ejaculated or reabsorbed by your body. This time period is anticipated to be the same for the IVD procedure, as well. Therefore, until you have two consecutive semen samples tested and they both show a zero sperm count, you are advised to practice protected intercourse (e.g., condom, IUD, oral contraceptives, etc.) by the method of your choice or it may be possible to conceive a pregnancy.
If the IVD implant is successful, you will return to the clinic at 2 weeks and again at 4 weeks after your procedure to be examined.
You will then be required to submit monthly semen samples, by dropping off the samples to the lab, for up to a total of 6 months. A special container will be provided to you by the clinic to obtain each sample. You will need to masturbate and collect the entire ejaculate in the container, record the date and time of collection on the container, and deliver the container to the lab or clinic on the same day it is collected. Further instructions on collecting, storing, and transporting the semen sample will be given to you by your laboratory and/or clinic. The sample will be analyzed to test if the IVD has been successful at blocking your sperm.
Once your samples have shown that your sperm has successfully been blocked for two consecutive months, the clinic will contact you to let you know that it is no longer necessary to bring in the monthly semen sample. The clinic will contact you by telephone 6 months after your last semen sample and again at 12 months to collect information regarding any additional contraceptive methods used and any effects related to your procedure. This interview is expected to take less than 30 minutes.
At 24 months, you will need to be seen in your study doctor for a final physical exam and to submit a semen sample, after which your participation in the study will end.
If the IVD cannot be implanted for any reason, a vasectomy will be offered to you at no cost.
Payment Schedule
If you participate, all of the procedures and devices related to the IVD will be provided to you without charge.
You will be paid for your time and inconvenience for participation in this study at a rate of $50 for each clinic visit or telephone follow-up that is required for this study.
If you complete all scheduled visits, submit all required semen samples, and follow the required time schedule, you will be paid an additional $200 after the 24 month follow-up visit.
Risks and Discomforts
The most frequent side effects, which may or may not occur, are the same as those associated with a vasectomy. These include:
fainting or seizures during the procedure
infection involving the testicles or scrotum
damage to the vas deferens
bleeding and/or pain at the site of incision during or after the procedure
Although rare, there are other risks of which you should be aware. These risks are similar to other vasectomy procedures which include:
unplanned hospitalization
persistent or significant pain in scrotum
other significant persistent or permanent physical or psychological harm or disability
death
unplanned pregnancy either due to failure of the IVD or not following the study doctor�s orders.
You should also be aware there may be other risks involved in this study that are unanticipated.
If you do experience any side effects, your study doctor will attend to you with any necessary examinations or treatments until the side effect resolves or stabilizes.
Disclaimer
The IVD has not yet been proven safe and effective by the FDA. Your doctor cannot guarantee that the IVD procedure will be successful at blocking your sperm and thus bring about sterilization.
Your participation in this study will help to determine whether the IVD procedure is safe and effective.
Study Clinic Locations
St. Paul,
Minnesota
Sartell, Minnesota
Shreveport, Louisiana
Tampa, Florida
If you would like to proceed,
1. Please review the informed consent available here as a PDF file.
2. Review the Study "Confidentiality & Privacy Rights" available here as a PDF file.
3. Call our office at 813-972-1365 and express your interest in participating in the IVD Study.
